

A lithium-ion (Li-ion) battery is a type of rechargeable battery that relies on lithium ions to store and release energy. These batteries are renowned for their high energy density, which means they can store a lot of power without taking up too much space. Plus, they are capable of retaining a charge over long periods.
In contrast to lithium-ion batteries, disposable dry-cell batteries, like alkaline or zinc-carbon batteries, are designed for single use and cannot be recharged. While inexpensive and readily available, they typically have a lower energy density and are not environmentally friendly due to their disposal after a single discharge. Lead-acid batteries, another type of rechargeable battery, have been used for decades, particularly in automobiles and backup power systems. They are known for their high power output and relatively low cost. However, lead-acid batteries are significantly heavier and have a lower energy density compared to lithium-ion batteries, and their cycle life is also generally shorter.
Now, where do we encounter these little powerhouses? These days, it seems they’re involved in everything.
For starters, they power a wide array of consumer electronics, from smartphones to laptops, tablets, and even the fitness tracker on your wrist nudging you to take a few more steps today. Lithium-ion batteries are also the backbone of the electric vehicle (EV) industry. These batteries offer efficient energy storage, which translates into longer driving ranges between charges.
Of course, lithium-ion batteries are indispensable when you come to think of renewable energy. Think tapping into solar energy during the day and using that stored power even after the sun has set. Or harnessing the wind’s might and saving that energy for a calm day. Thanks to lithium-ion batteries, that energy is there when we need it most, so we can move closer to a future where clean, sustainable power isn’t just a dream.
What are the Components of a Lithium-Ion Cell?
A typical cell is built from the following components:
1.Cathode
The cathode is the positive terminal of the battery and is usually made from materials like lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC), lithium nickel cobalt aluminum oxide (NCA), lithium iron phosphate (LFP), or lithium titanate oxide (LTO). It plays a big part in determining how much voltage the battery can produce and how long it can keep our devices humming along.
2.Anode
The anode, the negative terminal, is typically composed of graphite. It stores lithium ions during the charging cycle. When it’s time to release that energy, it does so with grace and efficiency, making sure we get the most out of our battery’s capacity. It’s a perfect example of how balance and harmony come into play even in technology.
3.Electrolyte
The electrolyte is like the lifeblood of the battery, often made of a lithium salt dissolved in an organic solvent. This solution allows lithium ions to move between the cathode and anode, facilitating the energy transfer that charges and discharges the battery.
4.Separator
As for its name, the separator is a thin membrane placed between the cathode and anode. Its job is to keep these two sides from coming into direct contact, which would cause a short circuit. At the same time, it lets lithium ions flow through unimpeded. Basically, it maintains order in the midst of all that energy movement.
How Does a Lithium-Ion Cell Work?
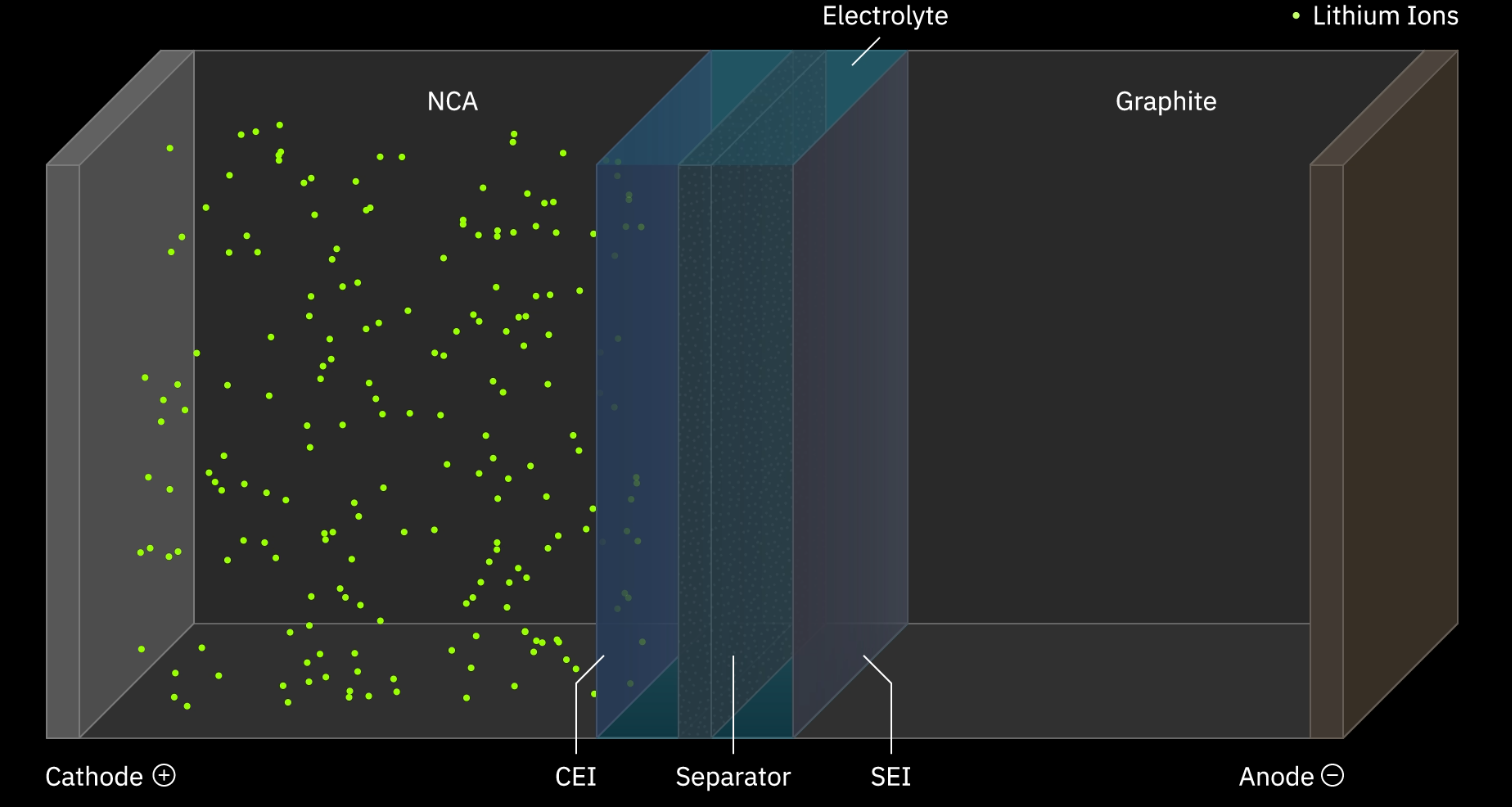
The working principle of a lithium-ion cell involves the movement of lithium ions between the anode and cathode through the electrolyte.
Charging
During this charging process, lithium ions are coaxed out from the cathode. These ions then make their way through the electrolyte and find a new temporary abode in the anode.
At the same time, electrons, which can’t move through the electrolyte directly because it’s an insulator, take a detour. They flow through the external circuit, your charging cable and device, to balance the charge. This flow of electrons is what we’re really after because it’s how the battery stores energy. When they arrive at the anode, they make sure all the lithium ions are nicely settled, creating a sort of energy storage.
Discharging
In the discharging cycle, the lithium ions, now well-rested and ready to get back to business, leave the anode and travel back to the cathode through the electrolyte.
As these ions move, electrons once again take the longer route through the external circuit. But instead of just balancing the charge, these electrons power up your device. This flow of electrons is what generates electric current, resulting in your phone screen lighting up, your laptop buzzing to life, or your electric car zipping down the road.
The Advantages of Lithium-Ion Cells
Lithium-ion cells come with a suite of benefits that make them highly desirable for various applications.
High Energy Density:
One of the primary advantages of Li-ion batteries is their high energy density, which allows them to pack a huge amount of power in a compact design. This quality is instrumental for applications where size and weight matter. Molicel’s ultra-high-power cell has long been renowned for its high energy density. Molicel’s INR-21700-P50B, equipped with a high-nickel layered oxide cathode, delivers an impressive energy density of 265 Wh/kg. This makes it ideally suited for motocross bikes and 2-wheeled motorcycle racing, contributing to exceptional horsepower and a lighter vehicle for ultimate speed. In comparison, lead-acid batteries typically offer energy densities ranging from 30-50 Wh/kg, making Li-ion chemistries like NMC and NCA roughly 5-8 times more energy-density. LFP batteries generally offer energy densities in the range of 100-160 Wh/kg, still significantly higher than lead-acid but lower than some high-nickel based batteries.
Low Self-Discharge:
Li-ion batteries exhibit low self-discharge rates, meaning they can maintain their charge for extended periods when not in use. This feature ensures that devices remain operational over longer standby times. Lead-acid batteries typically have a self-discharge rate of around 5% per month or higher, whereas Li-ion batteries (including NMC, NCA, and LFP) generally self-discharge at a rate of only 1-3% per month, offering a significant advantage in charge retention.
Low Impedance:
These batteries have low internal impedance, which translates to high energy efficiency. Low impedance reduces energy losses and improves the overall effectiveness of the energy storage system. Molicel has successfully reduced the maximum internal resistance of the INR-21700-P45B to an exceptionally low impedance of less than 13.8 mOhm. This remarkably low impedance contributes to an extended flight range for drones and eVTOLs. In general, NMC ,NCA and LFP batteries exhibit low internal impedance, typically in the range of 10-30 mOhm, contributing to their high power output capabilities. In contrast, lead-acid batteries typically have significantly higher internal impedance, ranging from tens to hundreds of mOhm depending on their type, size, and condition. This higher impedance leads to greater energy losses during charging and discharging and limits their high-power performance compared to NMC, NCA, and LFP batteries.
Fast Charging:
The ability to charge quickly is a big advantage for many modern applications. Molicel’s INR-21700-P50B, for instance, highlight their capability with a 5C fast charging capability (only 12 minutes), making them ideal for high-demand applications. While some advanced lead-acid batteries can handle relatively high charge rates, they generally cannot match the fast-charging capabilities of many Li-ion chemistries like NMC and NCA, which can often be charged at rates of 1C or higher. LFP batteries also offer good fast-charging capabilities, often exceeding that of lead-acid. Fast charging capability will minimizes downtime, ideal for hyper EVs and racing cars, ensuring your racing car returns to the circuit quicker.
Long Cycle Life:
Lithium-ion batteries boast a long cycle life, meaning they can go through many charge-discharge cycles before their performance degrades significantly. This durability makes them cost-effective over the long term. For example, Molicel’s Ultra-High Power Cells are noted for their exceptional cycle life with consistent performance, ensuring reliability and lengthened usability. Nickel-based batteries can typically achieve cycle life in the range of 700-1000 of cycles.LFP batteries are known for their even longer cycle life, often exceeding 3000-5000 cycles under proper conditions. In comparison, lead-acid batteries typically offer a cycle life in the range of 500-1500 cycles, making Li-ion chemistries, especially LFP, significantly more durable over repeated use.
Undoubtedly, lithium-ion batteries are a pivotal technology in our daily lives and future developments. That’s why understanding their components, operation, and advantages helps us appreciate their widespread use and continued innovation. They power everything from our beloved smartphones to life-saving medical equipment.
For those seeking robust and reliable energy storage solutions, explore Molicel’s Ultra-High Power Cell. Whether it’s for demanding industrial applications or cutting-edge consumer electronics, Molicel’s expertise and superior performance can meet your needs.
So, what are you waiting for? Take your energy solutions to the next level! Engage with a brand that understands the nuances of your energy requirements. After all, choosing the right battery is about more than just power—it’s about support, reliability, and the confidence to power the moments that matter most to you.


